Soil Pollution
Types and Sources of Pollutants:
We are talking about the very important topic of Soil Pollution. But to begin with we need to know something about the types and sources of pollutants. I could have spent hours on the internet searching for information on this topic but some of this is very complicated and too important to rely solely on searches on the web.
Let’s have a look at this picture. At the top of this picture you can see different types, different groups of types of pollutants. Imagine that alone in European environment more than 700 different pollutants are described. And if we distinguish them in three different groups we can distinguish organic ones, which can be persistent organic ones, which stay in the environment over many years or those ones, which underlay very short process of decay. For example the persistent organic ones, you may know DDT or others. Then, we have the inorganic pollutants. An example for this is heavy metals. And we have the new ones, so called particulate ones, which are nanoparticles and microplastics. We distinguish between urban sources, for example in the morning if you are standing under the shower and using specific shower gel then that may contain microplastics, and these are then released into the environment. We have, the next one, is agricultural activities. So for example application of pesticides or the application of veterinary drugs with animals. And the third one is industrial chemicals, which can be released by the chimneys or by industrial waste water.
A point source pollution results when pollutants come from a single location. An example for this is a pipeline, which is erupted, and then petrol comes out of this pipeline and contaminates a defined area.
Non-point-source pollution is when pollutants are introduced into the environment of a wide-spread area. One example for this is acidic rain, which has been a big problem in Europe in the 80’s, which were released by chimneys of the industry.
It’s about 1.3 million sides in Europe. So it’s an enormous side. And if we have a look here on this slide we can see that mainly waste deposition and treatment and industrial and commercial activities are responsible for this point source contamination. We can see the heavy metal distribution in European top soils. This is based on the Lucas database, which is a European database with more than 20.000 data points, resulting from a sampling in Europe. And you can see that we have exact maps about the lead distribution. You see the red area’s was high lead concentrations and the blue ones was low lead concentrations.
It is very obvious that with the rapid growth in the variety and quantity of pollutants in the environment at present, which is estimated at over 700 pollutants in Europe alone, that we have a problem in ensuring the long term health, productivity and sustainability of our soils. We need to fully understand the behavior of these pollutants, how they are mobilized and what risks if any do they pose to both our health and the health of our soils and ecosystems before we can effectively take action to reduce or remove the pollutant problem. But we urgently need to take action to address the issue to minimize any impacts from pollutants on our soils. This will ensure that we have healthy ecosystems and sustainable soil for life.
Organic Pollutants:
I'll explain pollutants pathways with the example of Persistent Organic Pollutants. You have industrial sources, urban sources as waste and traffic, and agricultural sources.
How can these pollutants then released from these sources be transported?
We have three general transportation ways. One is the air. This is a widespread, long range transport which can occur with the air. Pollutants, then, can be deposited by rain fall, snow, or by dry position as particulates into the soils. That means pollutants which are released, for example, in cities can be carried over thousands of kilometers to other places. An example of waste is the acid rain, which was a big of a problem in the 80's in whole Europe. The next important transport way is water. If waste water is not sufficiently cleaned, it ends up into the rivers and is there transported over thousands or hundreds of kilometers.
But what happens if this river water is used for irrigation?
Then it is directly deposited to agricultural soils. Or, if flooding occurs. The next, more direct pathway is land deposition. Pollutants can as well be directly deposited on land as part of fertilizers.
The irrigation, with contaminated water and as well the sludge application. Often sludge is used for fertilization of agricultural land, to increase organic matter. Not taking into consideration that it may contain organic pollutants or heavy metals. And what you can see here is the spraying of pesticides directly introduces pollutants into the soil environment.
How organic pollutants get into the soil and into our plants and also into the water in the soil?
Imagine these pollutants are deposited on the soil, it rains afterwards. What can happen if you have a smooth slope or a bigger slope, these pollutants can be transported with the rain water or the run off water and end up into lakes, rivers downstream. What as well can happen is that they can be transported in the soil water and can enter into the leaching processes in the ground water
and then pollute ground water bodies.
They can as well remain in the soils, depending on the properties of the pollutants, they can be strongly adsorbed by the soil and then they remain there for many years, depending on their decay.
Organic pollutants underlay (undergo) different decay and these decay can vary between several hours and decades of years. As well, what can happen is that these pollutants are photolyzed (photolysis), photolyzed by the light and then enter up in the air. Or they can be uptaken by plants. If these easiest plants are agricultural plants they can bio-accumulate in the food chain and if they enter into the rivers, then they enter in the aquatic food chain, they as well threaten human life.
It is very concerning that persistent organic pollutants or POPs can be spread from numerous diffuse sources and can affect all of us in so many ways within the soil-water environment. This can occur through the contamination of our groundwater sources, our surface water resources and they can accumulate and build up in the food chain. Ultimately, pollutants from these contaminated environments can end up in our food that we eat.
Therefore, there is an urgent need to manage diffuse sources of pollution more effectively at both national and international levels as the problem is not one that will simple go away. To ensure soil for life and our ecosystems well into the future, we must take action now to effectively manage POPs in our soil-water environment.
Inorganic Pollutants (Heavy Metals):
Heavy metals are inorganic pollutants, meaning that they are of mineral origin, not biological origin.
One definition of heavy metals is that they have a density of at least 3.5 gram per cubic centimeter.
Heavy metals cannot be degraded or destroyed. Examples of heavy metals include cadmium, copper, lead, nickel, and zinc. Heavy metals in soil can have a natural origin because they are present in the parent material from which the soil was formed. In many cases, these heavy metals are present in the crystal matrix of the finest soil particles, the clay particles.
As this picture shows the natural nickel content in undisturbed and non-polluted soils clearly increases with the amount of clay that is present.
This means that clays contain nickel. The same is true for most other heavy metals like zinc.
The heavy metal concentrations in soil that have a natural origin represent the so-called “natural background levels”. Because of human activity, additional heavy metals can enter the soil. This causes the heavy metal concentrations in soil to increase. When this happens, a soil can, strictly speaking, be considered polluted. It is important that you realize that elevated levels of heavy metals in soil do not necessarily lead to negative effects on soil life.
The effect of increased heavy metal concentrations depends not only on the severity of the heavy metal pollution, but also on the properties of a soil, like the amounts of organic matter, clay, and oxides and pH. These soil properties control the chemical behavior of heavy metals in soil,
and the extent to which soil organisms and plants are exposed to them. For this reason, these soil properties are important in determining heavy metal toxicity to soil life. Let’s now have a look at some specific cases of heavy metal pollution in agricultural soils. This picture shows rice paddy fields in Taiwan.
These fields are polluted with cadmium, because they were irrigated with untreated wastewater from cities and industry. Cadmium that is present in soil can be taken up by rice plants and accumulate in the rice grains. In this way, cadmium enters the food chain via the soil and leads to human health risks. This picture shows a field with so-called “copper balls” in China.
These copper balls are a mixture of electronic waste components and clay. They are burned in small ovens by local farmers to recover valuable metals from the electronic waste components. However, during the smelting process in the oven, part of the heavy metals is released into the atmosphere
and deposited in the nearby surroundings. This of course leads to pollution of rice paddy fields and other soils in the area.
Sewage sludge is often land-applied to improve the fertility of agricultural soils. Sewage sludge is a by-product of the treatment of household and industrial sewage water. Even though sewage sludge does contain organic matter and nutrients, it can also contain heavy metals. When sewage sludge containing heavy metals is land-applied, those heavy metals enter agricultural soils. Heavy metals can also enter agricultural soils through application of mineral fertilizer and organic manure by farmers.
Usually, mineral fertilizers and organic manures contain only very small amounts of heavy metals.
But when applied continuously and in large quantities, heavy metals like cadmium eventually accumulate up to levels at which they can become a threat to soil life and food quality.
As a last example, I want to mention zinc smelters which are a source of heavy metal pollution in many places in the world.
This particular zinc smelter near Budel, in the south of The Netherlands, caused widespread soil pollution with cadmium, lead, and zinc in an area of about 350 square kilometres. This resulted in high heavy metal levels in the soil, crops, and groundwater. The natural background level of cadmium for the soil in this area is around 0.4 mg per kilogram of soil. This means that the soil is polluted. Another typical feature of soil pollution by smelters is the decrease in the soil heavy metal concentration with increasing distance from the smelter.
Behavior and availability of heavy metals in soil:
I'll explain the chemical behavior and bio-availability of heavy metals in soil. Most heavy metals like cadmium and lead are toxic for plants and do not have a known biological function. But some heavy metals, like copper, nickel, and zinc are micro-nutrients. This means that plants require small amounts of these metals to grow. But even these micro-nutrients can become toxic in soil, when they are present in excess. A key question is then: what causes the toxicity of such micronutrients and at what levels does this happen?
To determine the critical levels of heavy metals in soil at which they cause toxic effects,
“dose – response relationships” have been established. These relationships illustrate the effect of a pollutant on an organism, depending on the level of the pollutant in the soil.
This figure shows a hypothetical dose response relationship for copper, an important micronutrient, and crop yield: at low levels of copper in the soil, marked as area 1 in this curve, an increase in the soil copper concentration leads to an increase in crop yield. At these levels, copper is not toxic, but rather a limiting factor for crop growth, in area 2 of this curve, where copper levels in the soil are higher, the plant has sufficient copper to grow. So, a further increase in the soil copper concentration does not increase crop yield but does not lead to toxic effects either. In other words, the plant is able to deal with the copper that is present in the soil, in area 3, the soil copper concentration becomes so high that copper is toxic for the crop, causing a decrease in crop growth and yield.
Dose - response relationships are useful for identification of critical thresholds for toxic concentrations of heavy metals in soil. And these thresholds can be used in development of soil policy to protect soil life. However, the toxicity of a heavy metal does not depend on how much of it is present in the soil alone; it also depends on the chemical behaviour of the heavy metal.
Once present, most heavy metals will bind strongly to soil components like organic matter, clays, and oxides. Only a minor part, usually less than 1%, will be present in the soil solution. This is important because plants take up heavy metals from the soil solution and not so much from the soil itself. This means that only the fraction of a heavy metal that is dissolved in the soil solution is available for uptake by plants. This applies to most soil organisms, like bacteria and fungi, as well. That is why we call the heavy metal fraction in the soil solution the “bioavailable” fraction.
So, the bioavailability of a heavy metal depends more on its concentration in the soil solution,
than on its total amount in the soil. The next question is then:
what determines the heavy concentrations in the soil solution?
Obviously, when the total amount of a heavy metal in the soil increases, its concentration in the soil solution usually increases as well. But the composition of soils is also important. When a soil contains more organic matter, a greater amount of the heavy metals will be bound to that organic matter. This means that the concentration of a heavy metal in the soil solution of a soil with 8% organic matter will be lower than that in a similar soil with only 4% organic matter.
Another important soil property in controlling the heavy metal concentrations in the soil solution is the pH.
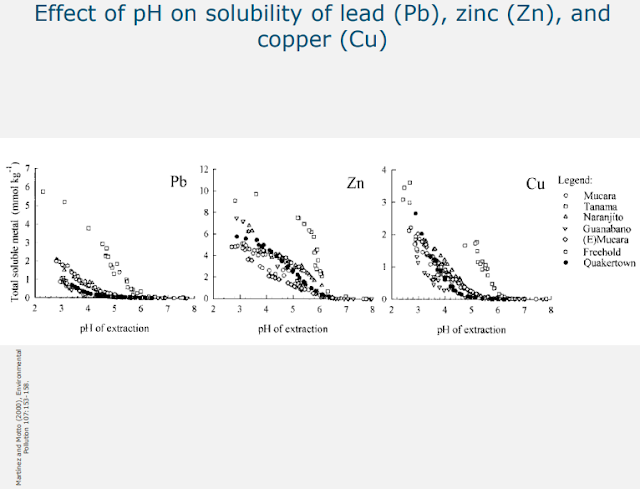
As you can see in this figure, reading the x-axis from the right to the left: as the pH of the soil decreases, the solubility of lead, zinc, and copper clearly increases. This means that the lower the pH of the soil, the higher the bioavailability of the heavy metals. I will illustrate the role that pH plays in and toxicity by looking at copper in the following example. In this picture, you see four pots: they are all filled with the same clay soil.
The amount of copper that was added to each pot is either low or high, and the same applies to the pH. Let’s first have a look at the second pot from the right, the one with a high pH and a low soil copper concentration. The grass looks green and healthy, meaning that the grass did not suffer from the low amount of copper in the soil. When we decrease the pH from high to low, the grass still looks green and healthy. This indicates that, even though the pH is low, the bioavailability of copper did not reach the level at which it is toxic for the grass. This is due to the fact that there is only a small amount of copper in the soil. Let’s now have a look at the pot on the right that has a high pH and a high soil copper concentration. The grass looks, again, green and healthy. This means that the grass is able to deal with the high amount of copper that is present. And that is because the bioavailability of copper was not so high that the copper was toxic. The reason is that the high pH kept the bioavailability of copper at such a low level that it did not cause any toxicity.
However, when we change the pH from high to low, the grass clearly suffers from copper toxicity:
it is yellow, and its growth is very poor when compared to the other pots. So, due to the low pH in combination with high soil copper concentration, the bioavailability of copper became so high that copper was toxic for the grass.
Remediation Techniques for Heavy Metal Polluted Soil:
When a soil is polluted with heavy metals to such an extent that they are a threat to soil life and human health, the soil needs to be remediated. The most comprehensive technique for remediation of a heavy metal polluted site is to excavate the polluted soil, and replace it with clean material. The excavated soil is disposed of safely elsewhere. As an alternative to disposal, the excavated soil can be treated on-site to separate the heavy metals from the soil. To achieve this, the excavated soil is placed in a pile or in a chemical reactor, so that it can be treated with chemicals that wash the heavy metals from the soil particles. The cleaned soil can then be re-used on the same site. One disadvantage of soil washing is that the chemicals used to clean the soil can have a negative impact on the chemical and biological quality of that soil, thus limiting its potential for re-use.
Instead of excavating the polluted soil, in-situ remediation techniques can also be used. In-situ means that heavy metals are removed from the soil without soil excavation.
A major advantage of in-situ techniques is that not only are costs for excavation and off-site transport reduced, but also disturbance of the soil is kept to a minimum. One example of an in-situ technique is electrokinetic remediation. In this picture, you can see how it works.
Electrokinetic remediation relies on electrode pairs that are placed in the ground, on each side of the polluted zone. When a low-intensity electric current is applied, positively charged cations of the heavy metals will move in the direction of the negatively charged electrodes, where they can be removed from the soil. A major disadvantage of both ex-situ and in-situ techniques is that they are expensive, especially when they are used to remediate large areas. This means that these techniques are mostly applied in smaller polluted urban areas where the urgency to remediate the soil is high in order to prevent health risks for people living nearby.
To remediate large areas, phytoextraction is a technique that offers a less expensive and possibly equally effective alternative. What is phytoextraction? Phytoextraction uses plants to remove heavy metals from the soil. As you can see in this picture, heavy metals are taken up from the soil by plant roots and partially translocated into the above-ground shoots.
By harvesting the plant shoots year after year, heavy metals are gradually removed from the soil. The harvested biomass can be safely disposed of elsewhere, or used as biofuel to generate energy. In some cases, valuable metals stored in the harvested biomass can be recovered and re-used. Advantages of phytoextraction are that it is inexpensive and that it is green: it makes the landscape more attractive. The vegetation cover that is established during phytoextraction also reduces wind erosion of fine soil particles with heavy metals. Moreover, moisture uptake by plants reduces runoff and leaching of heavy metals to ground- and surface waters.
Phytoextraction also has disadvantages. Since its effectiveness relies on uptake by roots, it can only be applied to soils where the polluted zone is localized in the topsoil where plants roots grow. Another disadvantage is that it takes a long time to complete remediation. The exact time that is required to clean the soil depends not only on the amounts of heavy metals to be extracted from the soil but also on the bioavailability of those heavy metals. Last but not least: the amount of heavy metals that can be extracted from the soil depends on the capacity of the plant to accumulate heavy metals and on the above-ground biomass produced each year. To increase the effectiveness of phytoextraction, plants are needed that are able to accumulate very high amounts of heavy metals in the above-ground biomass. So-called “Hyperaccumulators” can do this.
Hyperaccumulators are plants that can grow in soils with high heavy metal concentrations.
Hyperaccumulators are also able to translocate the heavy metals taken up by roots to the above-ground biomass that can be harvested. One example of a hyperaccumulator is Thlaspi caerulescens.
This plant species is found at scattered sites, all over Europe. Here you can see Thlaspi caerulescens in one of our phytoextraction experiments.
In this experiment, it is growing in a cadmium- and zinc-polluted sandy soil. An ecotype of Thlaspi caerulescens was used that originates from cadmium- and zinc-rich soils in the south of France. This ecotype can hyperaccumulate both cadmium and zinc. A common property of many hyperaccumulators is the limited amount of yearly biomass production in the field. This means that despite the high cadmium and zinc levels we found in the above-ground shoots of Thlaspi caerulescens in our phytoextraction experiment, the time required to reach the desired lower cadmium and zinc levels in the soil was much longer than ten years.
Another challenge that needs to be resolved is the limited knowledge of how to successfully farm hyperaccumulators under field conditions. Also, hyperaccumulators tend to accumulate only one or two specific heavy metals. As a result, there is very little or no extraction of other heavy metals that may be present in the same soil. All this means that before phytoextraction can be applied in practice, further developments are needed.
















Comments
Post a Comment